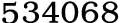|
A white crystalline solid, KHCO3, soluble in water and insoluble in ethanol; r.d. 2.17; decomposes about 120°C. It occurs naturally as calcinite and is prepared by passing carbon dioxide into saturated potassium carbonate solution. It is used in baking, soft-drinks manufacture, and in CO2 fire extinguishers. Because of its buffering capacity, it is added to some detergents and also used as a laboratory reagent. The compound is used as a source of carbon dioxide for leavening in baking, extinguishing fire in dry chemical fire extinguishers, acting as a reagent, and a strong buffering agent in medications. It is used as an additive in winemaking and as a base in foods and to regulate pH. It is a common ingredient in club soda, where it is used to soften the effect of effervescence. Potassium hydrogencarbonate is used as a fire suppression agent ("BC dry chemical") in some dry chemical fire extinguishers, as the principal component of the Purple-K dry chemical, and in some applications of condensed aerosol fire suppression. It is the only dry chemical fire suppression agent recognized by the National Fire Protection Association for firefighting at airport crash rescue sites. It is about twice as effective in fire suppression as sodium bicarbonate. Potassium hydrogencarbonate is an effective fungicide against powdery mildew and apple scab, allowed for use in organic farming. Potassium hydrogencarbonate is often found added to bottled water to affect taste. Potassium hydrogencarbonate has widespread use in crops, especially for neutralizing acidic soil
Related Articles -
Potassium hydrogencarbonate, 298-14-6,
|